Enhanced TDS
Identification & Functionality
- Chemical Family
- Chemical Name
- Ingredient Origin
- Animal Feed & Nutrition Functions
- Food Ingredients Functions
- Pharma & Nutraceuticals Functions
- Molecular formula
- C₆H₁₁NaO₇
- Technologies
- Product Families
Features & Benefits
- Labeling Claims
- Food Ingredients Features
Applications & Uses
- Markets
- Applications
- Animal Species
- Applicable Processes
- Food & Nutrition Applications
- Application Information
- It is predominately used for technical applications as an effective set retarder and plasticiser in concrete admixtures, as well as a chelating agent for calcium and magnesium ions in industrial, institutional and household cleaning products.
- It is also used for the cleaning of metal surfaces in the metal plating and electronic industry, and as formulations aid in agriculture to enhance micronutrient uptake.
- In personal care products, sodium gluconate is used as a chelating agent (replacement of EDTA) as well as a powerful moisturising ingredient.
- In the past years sodium gluconate’s use in food applications has become more widespread.
- It is used to cover bitter flavours of high intensity sweeteners, mineral salts and caffeine in beverages.
Properties
- Physical Form
- Soluble In
- Appearance
- White to Tan, Granular to Fine Crystalline Powder
- Soluble in
- Water
- Sparingly soluble in
- Alcohol
- Insoluble in
- Ether
- Microbiological Values
Value Units Test Method / Conditions Total Plate Count max. 200 cfu/g Yeast Count max. 10 cfu/g - Mold Count max. 10 cfu/g - - Specifications
Value Units Test Method / Conditions Assay 99 - 101 % - pH (at 10% Solution, at 20°C) 6.5 - 7.5 - - Reducing Substances max. 0.5 % - Chloride Content max. 50 mg/kg - Sulfate Content max. 100 mg/kg - Arsenic Content max. 2 mg/kg - Granulation (at > 1.18 mm) max. 10 % - Granulation (at < 0.15 mm) max. 35 % - Solubility 600 g/l - Sodium Content approx. 10.6 % - - Heavy Metals
Value Units Test Method / Conditions Lead Content max. 1 mg/kg -
Regulatory & Compliance
Technical Details & Test Data
- Chelating Performance Analysis
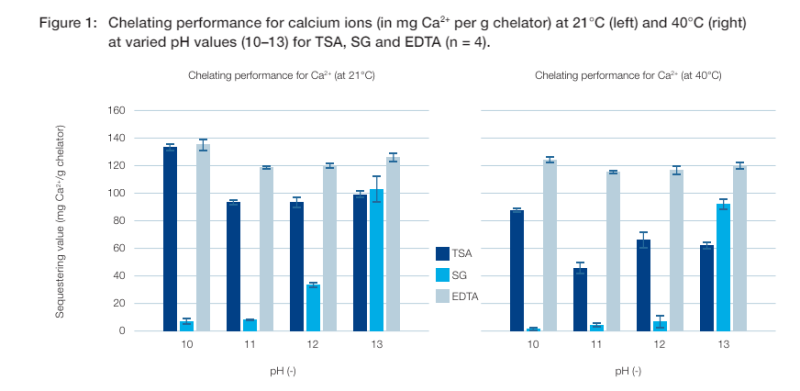
Sequestering values for calcium ions
The chelating performance for calcium ions was analysed using carbonate as the counterion (see figure 1), since this is especially relevant for home care applications. Measurements were carried out using a calcium acetate solution as the calcium source. The results for chelating capacity are shown in figure 1, with the left-hand column showing results at a temperature of 21°C, while the right shows results with an elevated temperature of 40°C.
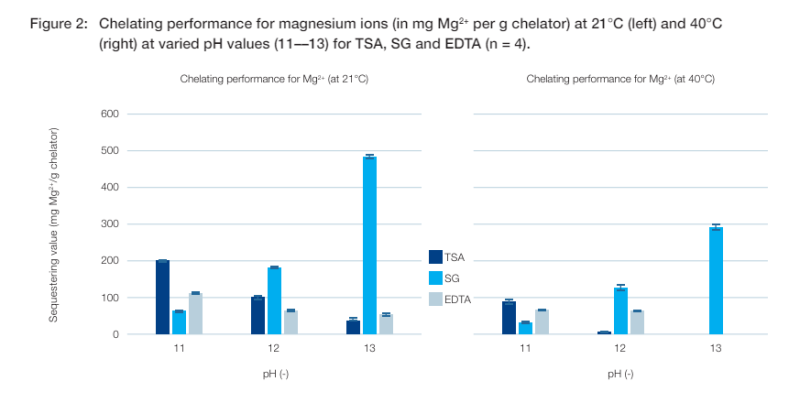
Sequestering values for magnesium ions
After calcium, the most common metal ions in tap water are usually magnesium. The method used for precipitation titration of magnesium was similar to that for calcium, but with magnesium acetate solution as titrant and hydroxide ions as counterions. Figure 2 shows the chelating performance for Mg2+ in a pH range of 11 to 13.
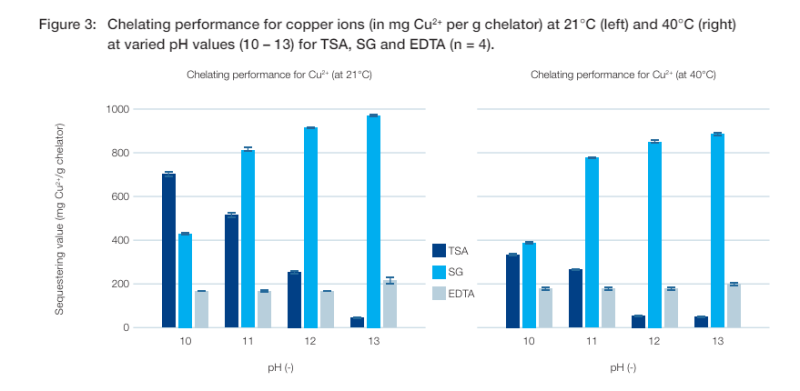
Sequestering values for copper ions
Chelating agents have the ability to complex transition metals like copper and iron, as well as calcium and magnesium ions. This behaviour improves the shelf life of the product, for example by optimising colour stability and delaying the oxidative degradation of oils and perfumes. 4 Additionally, building complexes with free trace metal ions protects the oxidising agents in laundry formulations, assuring their performance.
A copper sulfate solution was used as the metal ion source to determine chelating capacity for copper. Because of the ability of Cu2+ to form various complexes, the selection of a buffer system was not trivial. 3 It was therefore decided to adjust the pH value by adding sodium hydroxide alone. The available copper ions formed blue to turquoise complexes with hydroxide ions. The wavelength used to detect the precipitate was 470 nm.
Packaging & Availability
- Packaging Type
- Supplied by
- Packaging Information
- Jungbunzlauer sodium gluconate is available in 25 kg net polyethylene (LDPE) bags or in 1000 kg net polypropylene big bags.
